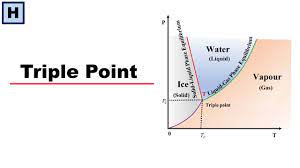
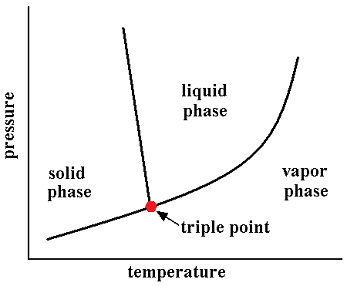
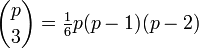In thermodynamics, the triple point of a substance is the temperature and pressure at which three phases (for example, gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. For example, the triple point of mercury occurs at a temperature of -38.8344 °C and a pressure of 0.2 mPa.
In addition to the triple point between solid, liquid, and gas, there can be triple points involving more than one solid phase, for substances with multiple polymorphs. Helium-4 is a special case that presents a triple point involving two different fluid phases (see lambda point). In general, for a system with p possible phases, there are

triple points.
The triple point of water is used to define the kelvin, the SI base unit of thermodynamic temperature.[2] The number given for the temperature of the triple point of water is an exact definition rather than a measured quantity. The triple points of several substances are used to define points in the ITS-90 international temperature scale, ranging from the triple point of hydrogen (13.8033 K) to the triple point of water (273.16 K).
Triple point cells
Triple point cells are used in the calibration of thermometers. For exacting work, triple point cells are typically filled with a highly pure chemical substance such as hydrogen, argon, mercury, or water (depending on the desired temperature). The purity of these substances can be such that only one part in a million is a contaminant, called “six nines” because it is 99.9999% pure. When it is a water-based cell, a special isotopic composition called VSMOW is used because it is very pure and produces temperatures that are more comparable from lab to lab. Triple point cells are so effective at achieving highly precise, reproducible temperatures, an international calibration standard for thermometers called ITS–90 relies upon triple point cells of hydrogen, neon, oxygen, argon, mercury, and water for delineating six of its defined temperature points.
The article content of this page came from Wikipedia and is governed by CC-BY-SA.
Like this:
Like Loading...